
Ayurvedic Nutraceutical CDMO in India
Formulation, GMP manufacturing, compliance, and export-ready production for Ayurvedic and herbal nutraceutical products.

how we can help
We’ll ensure that you focus on the opportunities
End-to-end programs.
Lorem ipsum dolor sit amet, consectetur adipisicing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat.
Support Functions
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis.
Service Operations
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis.
Supply Chain
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis.
Who We Are
Bliss Life Sciences LLP is an India-based Ayurvedic Nutraceutical Contract Development and Manufacturing Organization (CDMO) supporting wellness brands with formulation development, scalable manufacturing, regulatory compliance, packaging, and export-ready supply.
The company integrates traditional Ayurvedic ingredient systems with modern nutraceutical manufacturing standards for domestic and international markets.
- 200+ Active Brand Partnerships
- 850+ Formulations Developed
- 12+ Dosage Formats
- Multi-Country Export Presence
Operational Challenges We Address
- Regulatory complexity across AYUSH & FSSAI
- Lack of standardized Ayurvedic formulations
- Inconsistent product quality
- Difficulty scaling manufacturing
- Export documentation barriers
- Limited in-house R&D expertise
Our CDMO Services
Formulation & Development
- Custom Ayurvedic nutraceutical formulations
- Standardized herbal extract systems
- Functional blend design
- Stability & compatibility testing
- Dosage optimization
Manufacturing & Quality
- Pilot and commercial batch production
- GMP-certified processes
- Batch validation systems
- In-process and final QC
- Certificates of Analysis (COA)
Regulatory & Supply
- FSSAI & AYUSH alignment
- Product specifications
- Stability documentation
- Export compliance
- Packaging & logistics
Numbers
Client results
Brand Segments We Support
Startups & Emerging Brands
- Pilot batches
- Flexible MOQs
- Regulatory guidance
Growing Wellness Brands
- Line extensions
- Portfolio expansion
- Scalable manufacturing
Global Nutrition Companies
- Export manufacturing
- High-volume production
- Multi-market compliance
Institutional Programs
- Corporate wellness
- Preventive healthcare initiatives
- Hospital nutrition support
QUALITY CERTIFICATES











Export Manufacturing Capabilities
- Export documentation systems
- Shelf-life & stability data
- International labelling formats
- Market-specific formulations
- Global packaging compliance

Trusted Excellence
Why Brands Partner with Bliss Life Sciences?
- Dedicated Ayurvedic and herbal manufacturing focus
- End-to-end CDMO operating model
- Flexible production systems
- Strong regulatory capabilities
- Scalable long-term infrastructure
Learn more
FAQs – Ayurvedic Nutraceutical CDMO
Yes. We work with client-owned, proprietary, or classical Ayurvedic formulations. Full confidentiality and IP protection protocols are followed throughout development and manufacturing.
Yes. We manufacture formulations aligned with classical references such as Charaka Samhita and Ayurvedic Pharmacopoeia of India (API), subject to regulatory approval and compliance.
We help adapt traditional Ayurvedic formulations for modern dosage forms, improved bioavailability, taste masking, stability, and global compliance.
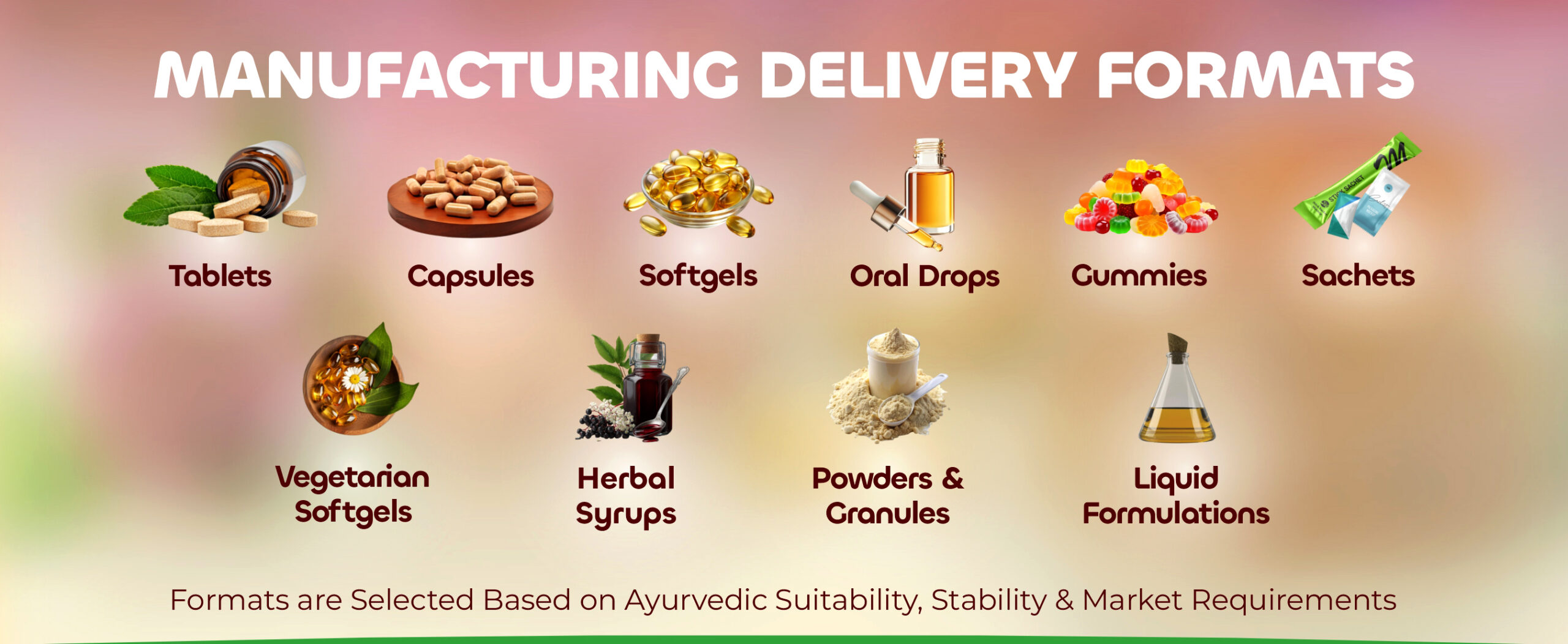
We offer:
- Tablets
- Capsules
- Powders & granules
- Syrups & liquids
- Effervescent formats
- Sachets
Yes. We use standardized herbal extracts wherever applicable, ensuring consistency in potency, marker compounds, and batch-to-batch quality.
Yes. All Ayurvedic products undergo mandatory testing for:
- Heavy metals
- Pesticides
- Microbial limits
- Aflatoxins
As per Indian and export-market regulations.
Yes. We assist with:
- Certificate of Analysis (COA)
- Free Sale Certificate
- Stability data
- Export-compliant labeling
- Country-specific regulatory documentation
Our manufacturing operations comply with:
- GMP
- ISO standards
- FSSAI
- AYUSH (where applicable)
- Export-quality compliance systems
Yes. We support pilot runs and flexible MOQs, making it suitable for startups, D2C brands, and market testing before scale-up.
Yes. Stability testing is conducted to determine shelf life, packaging compatibility, and storage conditions.
- Qualified raw material sourcing
- In-process quality checks
- Validated SOPs
- Batch traceability systems
Yes. We can develop formulations with minimal excipients, no artificial colors, and clean-label positioning where required.
Yes. Products can be tested through NABL-accredited third-party laboratories upon request.
Yes. We specialize in hybrid formulations combining Ayurvedic herbs with vitamins, minerals, and nutraceutical ingredients.
- Formulation finalization: 2–4 weeks
- Pilot & validation: 2–3 weeks
- Commercial manufacturing: 4–6 weeks



